STS News, Fall 2021 — This fall, the STS/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry will begin a much-anticipated public reporting program, where participating hospitals can demonstrate their ongoing commitment to quality measurement in the public eye.
“The same public reporting efforts that have powered the STS National Database will now be available for the TVT Registry, with the aim toward improving patient care,” said Vinod H. Thourani, MD, from Marcus Heart Valve Center at Piedmont Heart Institute in Atlanta, Georgia, who serves as vice chair of the Registry’s steering committee.
A collaboration between STS and ACC, the TVT Registry monitors patient safety and real-world outcomes related to transcatheter valve replacement and repair procedures. It collects and aggregates clinical data that give participants the evidence they need to understand their institution’s performance and consequently improve quality of care—as well as demonstrate success.
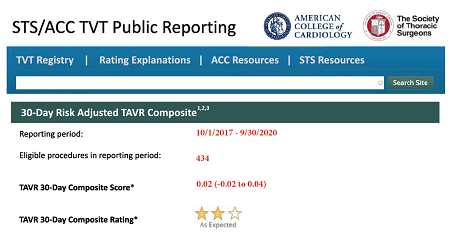
The new reporting platform will employ a “site win-difference” method to represent risk-standardized composite outcomes, both fatal and non-fatal, associated with transcatheter aortic valve replacement (TAVR) performed at each site.
The site win-difference model, used in clinical trials that have a composite of primary endpoints, provides different weights for adverse events surrounding valve procedures, including in-hospital or 30-day mortality, stroke, major bleeding, acute kidney injury, and paravalvular aortic regurgitation. Weights are based on the clinical importance and timing of the outcomes. This new method supplies the foundation of site reports, characterized publicly by a 3-star rating system that represents “better than expected,” “as expected,” or “worse than expected” outcomes.
In simple terms, star ratings are a visual representation of the probability that an average patient is better off going to a participant’s hospital versus an average hospital, minus the probability that an average patient is better off going to an average hospital versus the participant’s hospital.
“Public data submission is voluntary for participating sites, and because sites need 3 years of data in order to publicly report outcomes, it will take about 3 years before patient outcomes are fully represented,” explained Dr. Thourani. “Our goal is for transparency for TAVR care in the US.”
Endpoint variables were selected and ranked based on their adjusted association with 1-year mortality and the patient’s quality of life, self-reported via the Kansas City Cardiomyopathy Questionnaire (KCCQ). The TAVR 30-day morbidity/mortality composite, for example, includes 46 variables, including KCCQ scores and gait speed on a 5-meter walk.
The TAVR 30-day morbidity/mortality composite is a hierarchical, multicategory risk model that estimates risk standardized results, including the calculation of 1–3 stars for public reporting.
The initial consent period for TVT Registry participants to join the public reporting program closed in April 2021, and new consent periods will open on a yearly basis. Sites must have submitted a case to the TVT Registry prior to the first date in the reporting period, said David M. Shahian, MD, from the Division of Cardiac Surgery at Massachusetts General Hospital in Boston, who serves as co-chair of the TVT Public Reporting Committee and chair of the STS Work Force on Quality Measurement.
“You have to be a registry participant for 3 years,” Dr. Shahian said. “Your site must have performed 60 or more TAVRs within the 36-month reporting timeframe, and you have to have 90% or greater data completeness on the baseline—KCCQ and 5-meter walk—and then a status of ‘green’ or ‘yellow’ on data quality reports.” Thresholds for KCCQ and 5-meter walk test were relaxed for the second and third quarter of 2020 related to the impact of COVID at sites.
The reporting metrics include the date of a site’s first TAVR procedure, the cumulative volume at program inception, the average annual volume for the reporting period, and the site’s composite score and composite rating.
Participating in the Public Reporting initiative will give institutions advanced decision-making capabilities, driven by quarterly reports that show practice patterns, demographics, and procedure outcomes that compare their performance with that of the national experience. The interface will provide an executive summary dashboard that enables not only “big picture” review and assessments at a glance but also the ability to drill down and analyze outcomes on the patient level.
As with the STS National Database, participants will receive ongoing support from clinically experienced staff, and they’ll be able to participate in training activities and discussions via regularly scheduled webinars and other educational offerings.
The Public Reporting pages are expected to begin displaying results for current participants this fall, and public-facing pages will be available on the STS Public Reporting page. To learn more about the STS/ACC TVT Registry, visit sts.org/tvtregistry.