STS News, Winter 2022 — Audit results for the STS Adult Cardiac Surgery Database (ACSD) are set to be released in January 2022, and a Database audit policy now formalizes expectations for all Database components and helps participating sites complement their internal quality controls.
“It’s important to note the high bar we set for these data—sites are expected to have greater than 98% accuracy in outcome measures of operative mortality and major complications,” said Felix G. Fernandez, MD, MSc, who chairs the STS Workforce on National Databases and leads the audit initiative. “Early results show that the data managers have done an amazing job despite the challenges brought by COVID. Some were reassigned, many were working remotely or even furloughed.”
Through two external auditing partners, STS periodically evaluates data to ensure that patients, participants, stakeholders, and oversight bodies receive data that are accurate, complete, consistent, and high-quality, Dr. Fernandez said. “Right now, for the ACSD, we’re seeing greater than 97% agreement rates in the cardiac audit, which is phenomenal. And, with 97% of sites contributing across the country, it’s truly nationally representative.”
The Society also recently published the STS National Database Audit Policy, detailing expectations for all four Database components—not only to monitor accuracy but also to alert sites to any dips in accuracy and give them the guidance they need to get back on track. The policy doesn’t contain anything particularly “new,” Dr. Fernandez explained. “It has really just formalized the standards that STS has had in place for years.”
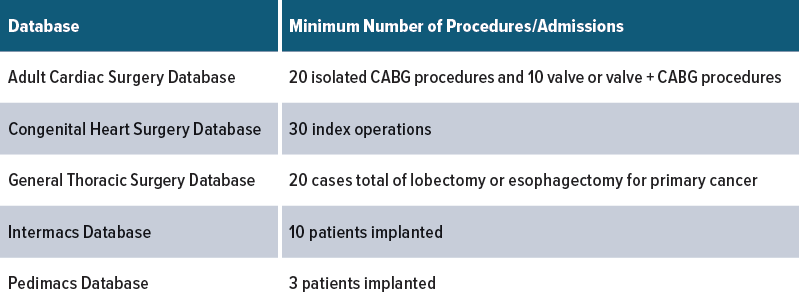
Audited sites must capture the minimum number for each procedure specified.
Each year, approximately 10% of participating sites are selected at random for audit. To be included in the audit pool, a site must be an active Database participant for all 12 months of the audited time period, have not successfully completed an audit in the past 3 years, and must have performed a specified number of procedures during that period.
“All the wonderful things that we do with the Database—risk models, research, performance measures that are reported to the public—are practice changing,” said Dr. Fernandez. “Data used for device surveillance impact policy on the national level. But these attributes are really of no value if the data are not high quality.” And this is why the audit is critical, he explained—the backbone of the Database is the quality of the data, and the assurances provided by the audit are what give the community confidence.
“We have made tremendous enhancements to the Database over the years, with a cloud-based platform, interactive dashboards, real-time access, and greater ease in data entry—and we have many more coming,” said Dr. Fernandez. “All these wonderful features help our participants improve their practice and make care safer for their patients.”
Read the published Database Audit Policy.