By Natalie S. Lui, MD, from Stanford University School of Medicine in California; and Sunil Singhal, MD, from the University of Pennsylvania in Philadelphia
STS News, Spring 2022 — Intraoperative molecular imaging (IMI) is a promising technology with several potential applications in thoracic surgical oncology.
The techniques involve an imaging agent, which is given intravenously and accumulates in the area of interest, and a fluorescence camera that detects the signal from the imaging agent intraoperatively. Imaging agents can be nontargeted or receptor targeted.
The ideal imaging agent is specific enough to produce a high mean fluorescence intensity (MFI) in the area of interest but not surrounding areas, yielding a high signal to background ratio (SBR).
The most commonly used nontargeted imaging agent is indocyanine green (ICG), the only FDA-approved near-infrared agent. At low doses, ICG imaging relies on vascular perfusion and has been used to define the intersegmental plane during segmentectomy, evaluate gastric conduit perfusion during esophagectomy, and identify the phrenic nerve during mediastinal mass resection.
At high doses, ICG relies on the enhanced permeability and retention effect and has been used for lung tumor identification; it is called TumorGlow to emphasize its higher dose and purpose. Clinical trials of IMI using ICG in patients undergoing surgery for lung cancer have shown good fluorescence signaling in tumors but also in peritumoral inflammation, demonstrating the advantage of receptor-targeted imaging agents.
Receptor targeted imaging agents are composed of a probe, which targets a receptor in tumors, conjugated to a dye, which fluoresces at a certain wavelength. For example, one IMI agent in clinical trials is OTL38 (On Target Laboratories in West Lafayette, Indiana). The probe is a folate analog, and the dye is S0456, a fluorophore in the near-infrared spectrum. The folate receptor is highly expressed in lung adenocarcinoma.
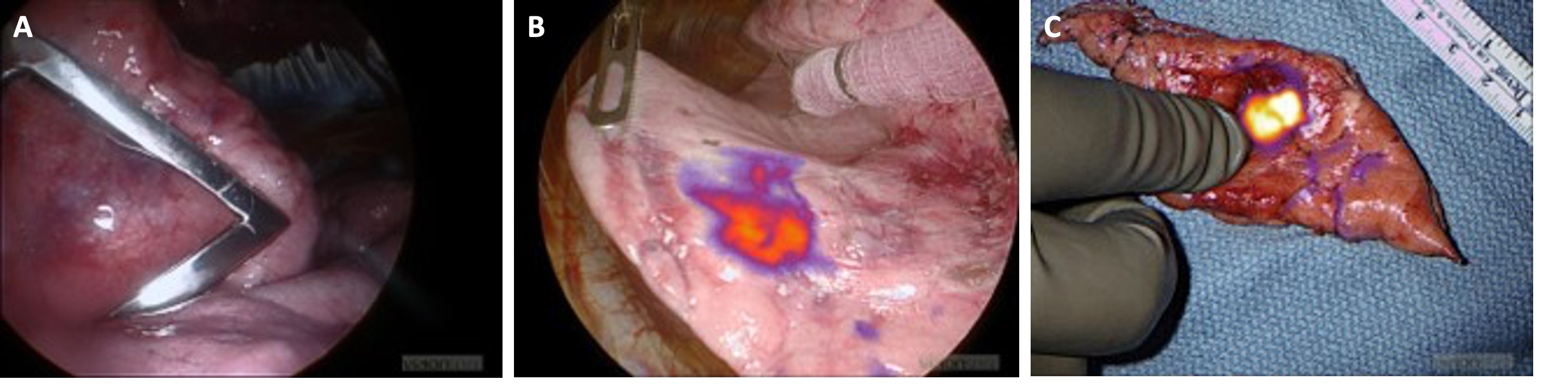
Another IMI agent being studied is panitumumab-IRDye800. The probe is panitumumab, an anti-epidermal growth factor receptor (EGFR) antibody, and the dye is IRDye800, another fluorophore in the near-infrared spectrum. EGFR is overexpressed in both lung adenocarcinoma and squamous cell carcinoma. Near-infrared dyes have been found to have greater depth of penetration compared to those in the visible spectrum.
One important application of IMI is tumor localization during sublobar resections, which are performed more frequently as we diagnose earlier stage disease. IMI techniques are particularly suitable for minimally invasive surgery, because the small incisions make it difficult for surgeons to palpate nodules. In addition, fluorescence cameras are built in to the newer endoscopic and robotic cameras and are easy for surgeons to learn.
There are several advantages to IMI tumor localization compared to our current methods of computed tomography (CT)-guided or bronchoscopic marker placement for lung nodules that are small, part solid, or deep to the pleural surface. IMI avoids an additional procedure before surgery and does not require radiation exposure to the patient or surgical team. The methods have been shown to be faster than intraoperative frozen section. Since IMI agents are given systemically, surgeons may be able to find synchronous lesions not identified on preoperative CT scans.
Additional research is needed before IMI is standard in clinical practice. There are currently no approved IMI agents, although OTL38 is currently being studied in a randomized clinical trial of patients with lung adenocarcinoma.
Development of probes that target different types of tumors, and allowing multiple probes to be used at once, would be important advancements. A current limitation is depth of penetration, and new fluorophores and fluorescence cameras are needed for reliable identification of deeper tumors. Another area of inquiry is evaluation of lymph nodes, which may be difficult to assess because they can take up the imaging agents even if not involved.
IMI is an exciting new technology with many potential applications for thoracic surgical oncology. Additional trials are needed before these techniques are approved for clinical use.