By Chandler A. Long, MD, and G. Chad Hughes, MD, from Duke University Medical Center in Durham, North Carolina
STS News, Winter 2022 — Endovascular repair of thoracoabdominal aortic aneurysm (TAAA)—a concept which seemed like pie in the sky less than a decade ago—is now an increasingly utilized technique in a growing number of centers worldwide, especially for patients with atherosclerotic TAAA.
To this point, a recent multicenter population-based study of patients undergoing TAAA repair between 2006 and 2017 in Ontario, Canada, found that endovascular repairs comprised >50% of all TAAA repairs in the province since 2011. Total endovascular approaches to TAAA repair have been developed to minimize the known risks associated with open repair and allow safer repair in the typical high-risk patient population presenting with atherosclerotic TAAA.
Although devices such as the Cook T-Branch stent graft, which is available outside the US, and the investigational Gore Excluder Thoracoabdominal Branch Endoprosthesis, which is currently being evaluated in a pivotal clinical trial, have been developed specifically for endovascular TAAA repair, neither is yet commercially available in the US.
This lack of a commercially available option has given rise to the use of so-called physician-modified endografts (PMEGs). PMEGs involve the modification of commercially available endografts, most commonly the Zenith Alpha and TX2 thoracic devices, whereby the devices are unsheathed in the operating room under sterile conditions and customized with reinforced fenestrations that correspond precisely to the patient’s visceral anatomy.
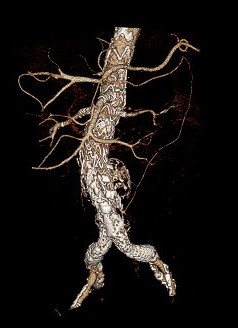
This 3D reconstruction CTA image shows a completed endovascular Extent IV TAAA repair using a four-branch PMEG device.
Temporary diameter-reducing ties are likewise created, allowing for partial deployment of the device without engaging the aortic wall, and thereby provide maneuverability of the endograft in cranial/caudal and rotational directions. Once the device has been fully modified ex vivo, it is then re-sheathed into its original delivery system and deployed endovascularly, subsequently allowing for delivery of branch stents grafts into the visceral vessels to complete the repair as described below.
The technical success of these procedures is contingent upon detailed preoperative planning using centerline analysis on a dedicated workstation. Although the details of case planning are beyond the scope of this brief report, the steps have been well standardized and enable the measurement of the exact coordinate locations of the target vessels to reflect the way in which the endograft is predicted to lie within the aorta due to any associated tortuosity.
The stent graft fenestration is performed on the back table by several members of the operating team during the induction of anesthesia and while other members of the team gain vascular access. We typically obtain open surgical access of one common femoral artery to allow delivery of the large bore PMEG delivery system, while percutaneous access of the contralateral femoral vessel is obtained using a “pre-close” technique.
Further, we prefer to introduce the branch stents antegrade, which is usually done via open surgical access of the infraclavicular left axillary artery. This access site allows the patient to be positioned with both arms at the side and gives more room for the operators positioned at the patient’s left shoulder to deliver the branch stents.
The fenestration holes are created in the stent graft using an ophthalmic cautery device and reinforced with a running locked 5-0 braided polyester suture incorporating a highly radiopaque gold gooseneck snare device wire.
Once the PMEG has been fully modified and resheathed, the device orientation with regards to the location of the fenestrations is checked under fluoroscopy and the device then partially deployed in the patient’s aorta down to the level of the celiac fenestration. The celiac fenestration is cannulated from above, and the device is then serially deployed to expose each fenestration for cannulation, adjusting the alignment of the PMEG device as needed to optimize target vessel alignment. This technique greatly simplifies the cannulation of the fenestrations and branch vessels.
Likewise, the use of fusion imaging allows the patient’s preoperative CTA imaging to be overlaid on the fluoroscopic image during the procedure, creating a three-dimensional map of the visceral vessel locations to assist with their cannulation and thereby minimizing contrast administration and fluoroscopy time.
Once all four visceral vessels have been cannulated, the PMEG device is fully deployed and the target vessels stented using balloon-expandable covered stents with the proximal ends of the branch stents post-dilated to flare them and prevent type III endoleak.
When this is complete, the seal zones of the PMEG device are post-dilated and the abdominal endografts, if they are being utilized, are then deployed using standard endovascular abdominal aortic repair techniques. Completion angiography is then performed to assess the patency of the visceral branches and rule out any endoleak.
In summary, endovascular repair is rapidly evolving to now becoming the first line option for most patients with atherosclerotic/degenerative TAAA outside of the connective tissue population. The technique has been demonstrated to be safe and effective and is transforming, for the better, the way TAAA is treated in the majority of patients.
To view videos that support techniques described in this article, visit the STS YouTube channel or scan the QR code below.
